PRELUD Project

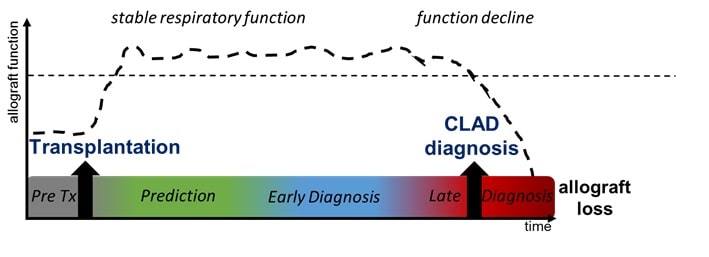
Chronic lung allograft dysfunction (CLAD) is the main limitation of long-term survival after lung transplantation. Although several risk factors of CLAD have been identified, pathophysiological pathways and molecular triggers remain unknown. Moreover, no integration of risk factors is performed in clinical practices. CLAD is diagnosed lately, upon persistent decline in lung function, revealing an advanced and irreversible degradation of the allograft at that stage.
Objectives
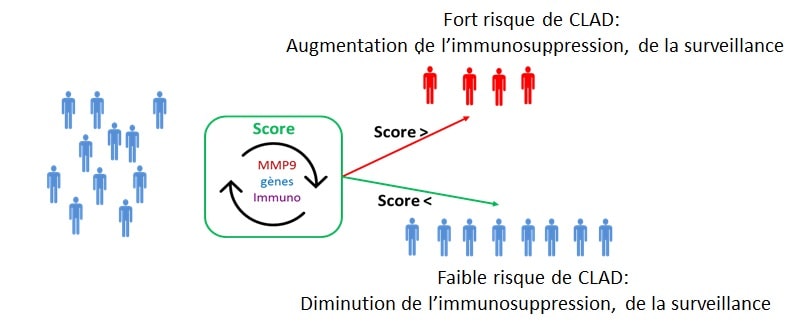
We have identified and validated several blood biomarkers able to predict CLAD more than 6 months before the clinical diagnosis. From these preliminary results, the primary objective of this project is to elaborate and validate a predictive and non-invasive composite (biological and clinical) score of CLAD. This score will be established at 1-year post-transplantation, a time far enough from peri-operative events that may non-specifically alter measured biological parameters but early enough to allow a safe and efficient management of CLAD before its onset This score will include pertinent clinical parameters and biological markers identified in our laboratory.
This score will be elaborated from samples from the COLT cohort (COhort in Lung Transplantation). Then, it will be validated on an independent set of 190 patients recruited for this PRELUD project.
This project is a translational research project that will open new fields of investigation for CLAD. It will provide clinicians with a robust, non-invasive clinical decision support tool, for a personalized follow up of the recipients. This project will thus be a major breakthrough in the management of lung transplant recipients.
Moreover, our preliminary data suggest an association between B cells and the appearance of CLAD. This hypothesis is supported by animal models and recent literature showing de novo donor-specific antibodies (DSA) and antibody-mediated rejection as a major risk factor for CLAD, with an impact on recipient survival. The secondary objective is thus to investigate the role of B cells and DSA in CLAD physiopathology. In a first task, multiparametric assays comparing CLAD with patients with stable respiratory function will decipher in details which B cell subset is altered in BOS and whether regulatory B cells are impaired in this setting. Then deeper investigation, using transcriptomic and functional analyses, will highlight whether an altered balance of regulatory B cells /inflammatory B cells or altered qualitative parameters of B cells trigger CLAD. A second task will be dedicated to exhibit the direct effect of DSA on the airway epithelium thanks to a culture model previously established. Moreover, this objective has also the potential to reveal molecular targets that can be modulated to favor regulatory B cells in a biotherapy.
In short, the PRELUD project will open new fields of investigation, beyond state of the art, for a better understanding of CLAD physiopathology, bringing potential therapeutic strategies. It will provide clinicians with a clinical decision support tool, for a personalized follow up of the recipients.
This project is funded by an ANR /DGOS research program, the Fonds de Recherche en Santé Respiratoire and the Institut de Recherche en Santé Respiratoire des Pays de le Loire (IRSR-PL ).
* Brosseau C et al. Blood CD9(+) B Cell, a biomarker of bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2019 Nov;19(11):3162-3175
Durand M et al. High circulating CD4(+)CD25(hi)FOXP3(+) T-cell sub-population early after lung transplantation is associated with development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2018 Jun;37(6):770-781.
Danger R et al. Blood Gene Expression Predicts Bronchiolitis Obliterans Syndrome. Front Immunol. 2018 Jan 11;8:1841.
Pain M et al. T Cells Promote Bronchial Epithelial Cell Secretion of Matrix Metalloproteinase-9 via a C-C Chemokine Receptor Type 2 Pathway: Implications for Chronic Lung Allograft Dysfunction. Am J Transplant. 2017 Jun;17(6):1502-1514.
