MELROSE Project

Context
Osimertinib, a third-generation tyrosine kinase inhibitor (TKI), is a new therapeutic option in advanced, non-pre-treated non-small cell lung cancer (NSCLC) expressing the EGF receptor mutation (EGFR).
The tumor escape mechanisms following first-line treatment with osimertinib are partially known, with most of the data obtained by analysis of circulating tumor DNA (cDNA) from the FLAURA Phase III trial.
Objectives
The MELROSE study, a French multicenter phase II trial promoted by the Nantes University Hospital, aims to treat 150 patients with a mutated EGFR NSCLC. All patients will receive osimertinib at a dose of 80 mg/d. Evaluation of response to treatment is performed by radiological examinations (thoraco-abdomino-pelvic and brain scans) every 3 months.
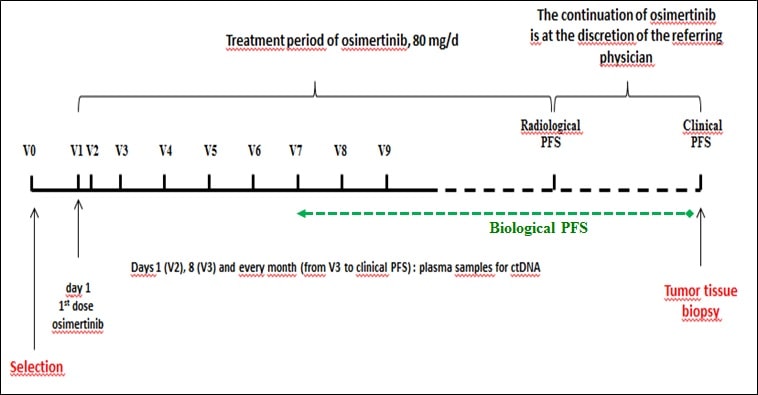
Continuation of osimertinib is at the discretion of the referring physician, particularly if a clinical benefit is observed.
The main objective is the genetic profile of the tumor, both on tissue biopsy and cDNA analysis, at the time of disease progression. Other parameters include cDNA kinetic studies, biological progression-free survival, median progression-free survival and median overall survival.
This study began in April 2019 and 18 French centers are participating.
This study allows the patient with non-mutated EGR NSCLC to benefit from osimertinib and it may be possible, through genetic analysis of the tumor, to possibly propose another targeted treatment in case of disease progression.
